Biospecimen Operations Support 70% of FDA Submission Data.
Why do Industry-Leading Practices Get It Wrong?
Slope orchestrates the entire biospecimen lifecycle — lab kits, samples, and metadata — before, during, and after collection.
We help sites achieve operational simplicity through standardized, flexible workflows already independently adopted by 2,200+ sites.
We help sponsors mitigate risk and accelerate timelines with solutions that 98% of their sites readily adopt.
We help CROs respond to site and sponsor needs at scale with a unified framework and toolset.
The Processes Every Trial Must Get Right
Every biospecimen goes through three critical processes. Here's what must happen — and why industry-leading practices consistently fall short.
Sites Must Be Prepared
Why industry-leading practices get it wrong: Sites have no easy way to track what supplies they have and where they are — made worse by overflowing storage closets packed with excess lab kits. Sites juggle excess inventory across limited storage areas with little to no systematic tracking. Central labs ship kits based on inaccurate forecasts, leading to expired supplies sitting unused while sites face emergency shortages. Protocol amendments cascade through updated documentation, leaving sites uncertain which versions apply. The result: 76% preventable lab kit waste and sites drowning in operational complexity.
What needs to happen and why: Sites need the right lab kits on hand to conduct a patient visit. Kit supplies must continuously align with actual patient demand and expiration dates. Lab manuals and protocol requirements must be clear, current, and consistent across all sites to ensure downstream compliance.

Sites Must Execute Flawlessly
What needs to happen and why: Sites must collect, process, store, and prepare samples according to precise protocol requirements to prevent deviations — capturing complete, accurate metadata at every step. When protocols are complex and requirements vary by patient, visit, trial, lab, and sample type, there's no room for error.
Why industry-leading practices get it wrong: Sites rely on paper/PDF lab manuals and requisition forms scattered across lab-specific portals and workflows. Research coordinators make judgment calls with incomplete information, leading to protocol deviations that surface days or weeks later. Your sites aren't failing; they're operating with tools designed for a different era.
.svg)
Samples (and Their Metadata) Must Reach Their Destination Intact
What needs to happen and why: According to ICH E6(R3) GCP requirements, every sample needs documented chain of custody from collection through final receipt. Samples must ship to the correct lab, at the right time, under proper conditions. Labs must receive complete, accurate metadata that matches what's in your EDC.
Why industry-leading practices get it wrong: You have no visibility into where samples are between site shipment and lab receipt. Was it shipped? To the right lab? On time? In the right condition? You discover problems only after samples are compromised — tracking numbers days later and metadata discrepancies weeks after sample receipt. A single late-phase oncology trial generates thousands of queries from missing requisition data or sample handling errors. You can't prevent what you can't see.
.svg)
Slope’s Approach: When Sites Succeed, Everyone Wins.
We knew there had to be a better way — one that recognized a simple truth: when you make sites successful, your entire trial benefits.
That's why Slope orchestrates your lab kits, biospecimens, and sample metadata from supply logistics to final sample receipt. Our platform gives site staff the tools they need to succeed while giving you the visibility and control you've been missing.
We built Slope because we've lived these frustrations firsthand. Our founding team includes former clinical research coordinators and clinical operations experts who spent too many Friday afternoons chasing tracking numbers, explaining sample losses to stakeholders, and drowning in preventable queries.
The data proves our approach works:
WHAT OUR CLIENTS say
"Slope not only helped us hit our enrollment targets faster than expected, but it also eliminated inventory and sample-related issues. The efficiencies we gained had a direct impact on the success of our study and endeared us to our clinical sites, who appreciated the convenience of automated sample management, manifests, and shipping labels."

"Our site receives and utilizes thousands of kits from our clinical trial sponsors. Slope allows us to organize our inventory by disease team, view our inventory anytime, any place, and quickly reorder low inventory. Supplying our sponsors with reports of current inventory is effortless with Slope."

“We needed a way to manage samples that didn’t involve piecing together data from disparate sources, only to find out that there were still gaps. Forall of our studies, Slope helps us mitigate risk by giving full insight into the status of samples at every stage of their lifecycle — from site collection to arriving at their destination. I would recommend Slope to any Phase I program with sample management needs!”
"Slope was invaluable when preparing for dose escalation meetings. Through filterable reports and real-time alerts, the software allowed us to pinpoint exactly what was missing and where we needed to go to find it so we could support dose escalation with a complete data set. Prior to Slope, this would have been a completely manual process for the team."
"Slope has more features and is more user-friendly than I anticipated. We are able to accomplish all of our goals by using the platform. The customer service is also phenomenal. Whether it's through an email or a phone call, the whole team is always happy to answer any questions that we have, or guide us through any support that we need."

the platform
One Platform.
Every Stakeholder.
Complete Orchestration.
Slope orchestrates your lab kits, samples, and sample metadata — with the ability to connect data from sites, labs, suppliers, couriers, and core clinical systems like EDC and RTSM — so nothing falls through the cracks. Whether you’re a site, sponsor, or CRO, we can even tailor our solution to the needs of your organization and/or trial(s).
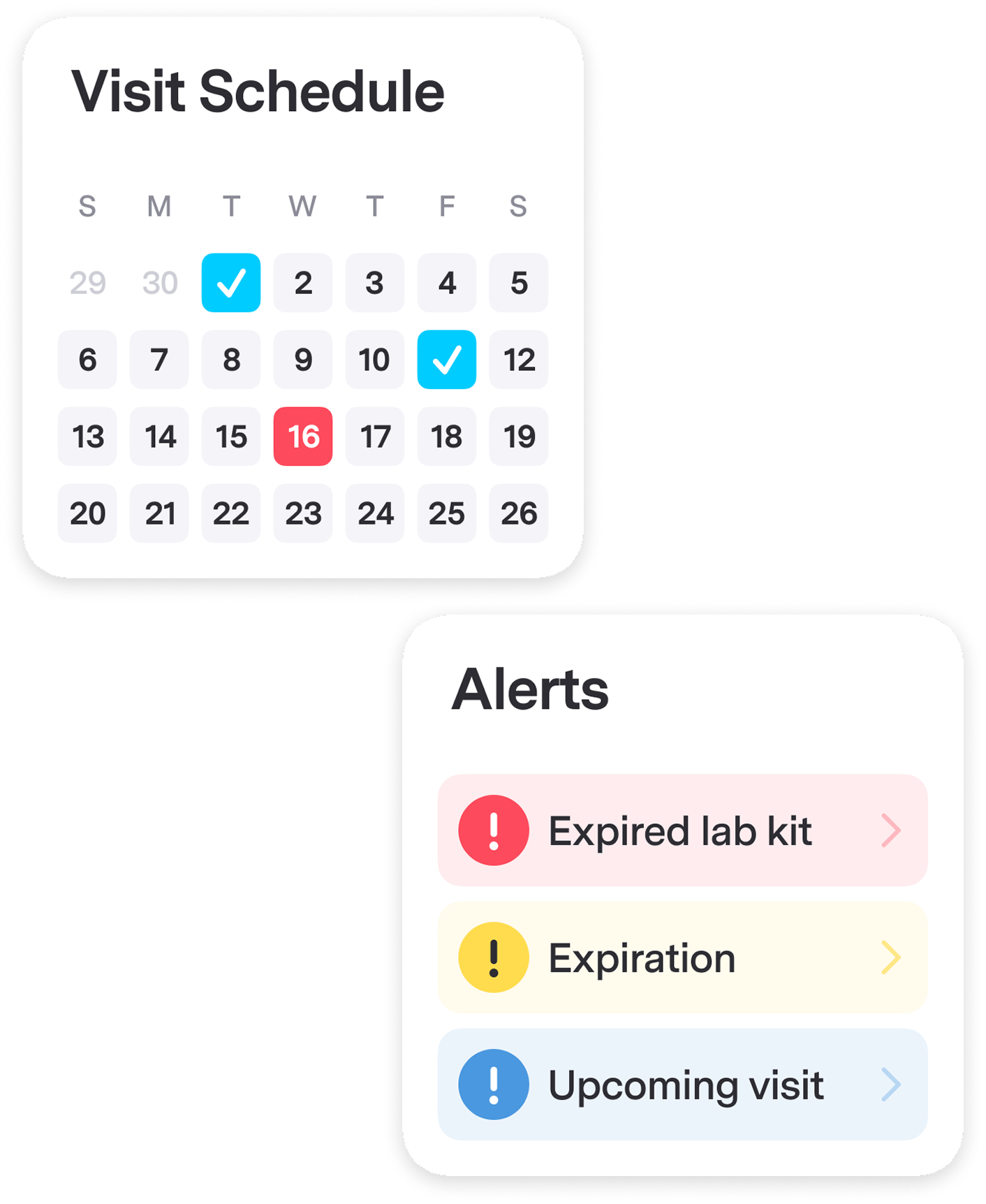
Sites Are Always Prepared for Patient Visits
Smart Lab Kit Management: Sites get intuitive tools with visit scheduling and automated tracking of storage locations, quantities, expiration dates, and waste. Data-driven resupply means sites always have what they need — eliminating waste and emergency shortages.
Protocol Amendment Management Protocol amendments cascade through updated documentation, leaving sites uncertain which versions apply. The result: 76% preventable lab kit waste and sites drowning in operational complexity. Protocol Amendment Management Seamlessly implement protocol changes on a site-by-site basis. Every site automatically works from the most current requirements, eliminating version confusion.
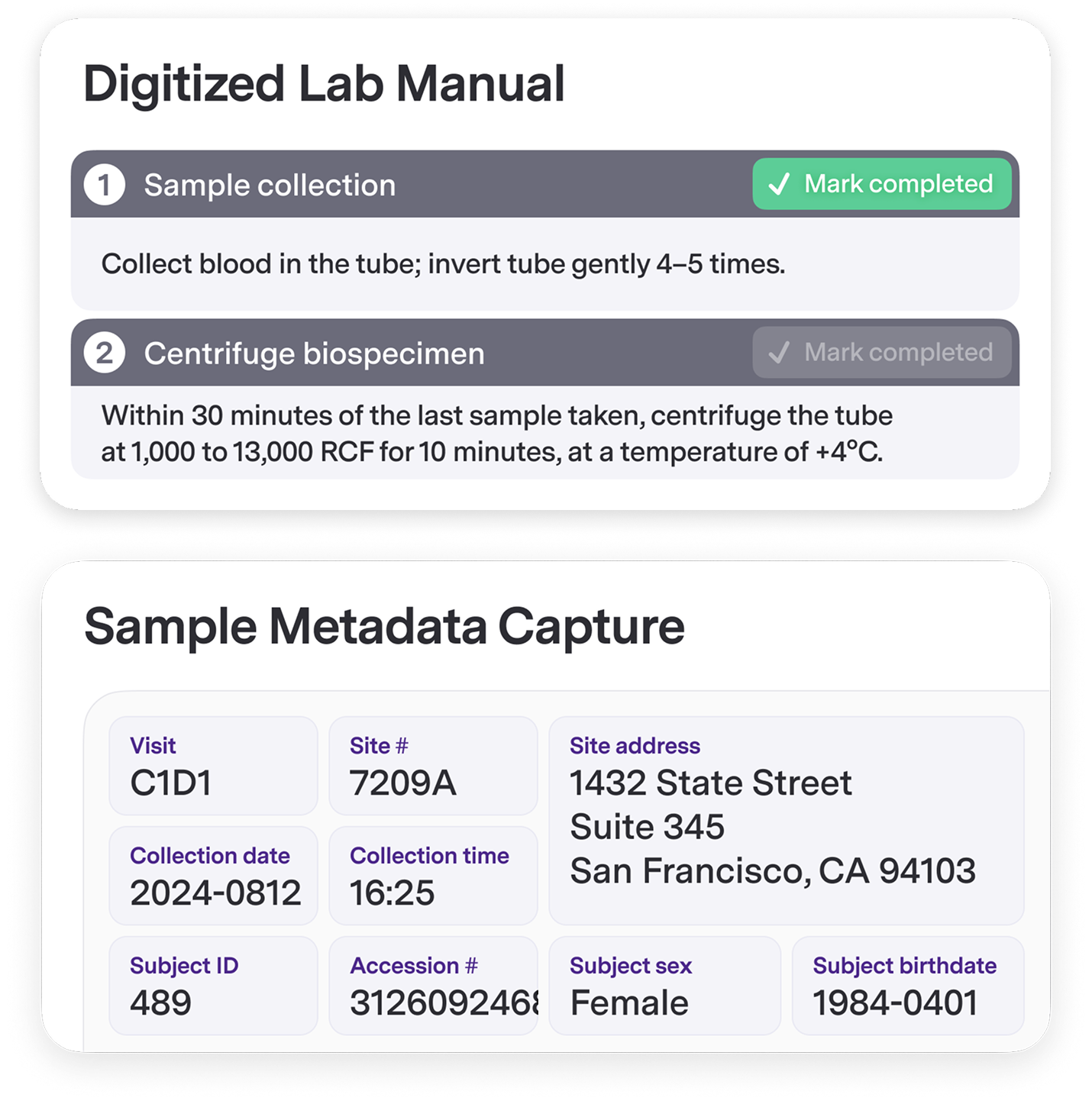
Sites Execute Flawlessly
Site-Friendly Guided Sample Workflows Replace static lab manuals with software-enforced guardrails for collection, processing, storage, and shipment. Sites get the flexibility they need with the compliance you require.
Compliant Metadata Capture Capture sample metadata through guided workflows that comply with SOPs and ALCOA+ principles. Automatically share data with labs and EDC — eliminating up to 98% of requisition queries and completely preventing lab-EDC discrepancies.
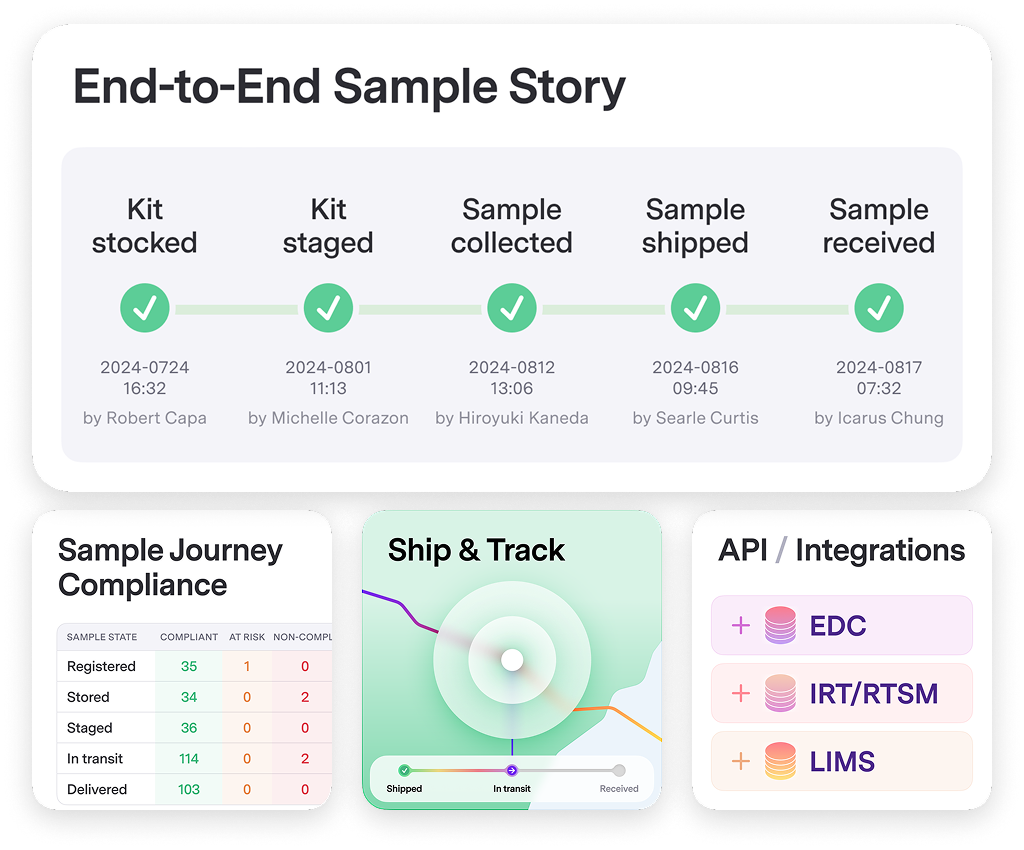
Sample Logistics Are Compliant and Metadata is Clean
Intelligent Shipment Coordination Automated shipping guardrails prevent wrong-lab errors and deliveries when labs are closed. Track all shipments with integrated courier data and instant alerts for issues.
Unified Stakeholder Platform Synchronize and exchange data across sites, labs, couriers, suppliers, and clinical systems — in one place. Everyone sees the same data, eliminating coordination chaos.
End-to-End Sample Tracking Monitor samples from collection through final destination with up-to-date dashboards, reports, and alerts. Know exactly where every specimen is, always.
the slope advantage
Your Path Forward
Take the First Step Towards Transforming Your Clinical Operations
Join industry leaders who trust Slope to transform their most critical trial asset — biospecimens — from one of their trials largest bottlenecks to one of their trial’s biggest operational advantages.





